Our Approach to Restorative Neurology & Neurorehabilitation

The Montefiore Einstein Division of Restorative Neurology is an internationally recognized leader in the fields of restorative neurology and neurorehabilitation and is dedicated to improving and restoring residual neurological function in adults and children with nervous system disorders.
Our multidisciplinary team of world-renowned specialists brings together research and clinical care, advancing the treatment of the full range of pediatric and adult nervous system disorders, including stroke and associated neurovascular conditions; multiple sclerosis and other demyelinating conditions; Parkinson’s disease and other movement disorders including dystonias, neuromuscular disorders and neurodegenerative diseases, pediatric and adult cognitive disorders; concussions and traumatic brain injuries; paralysis and spinal cord injuries; as well as the full spectrum of other nervous system conditions.
Our specialists apply newly emerging scientific discoveries in the field of neurorestoration, altering the course of the condition, restoring function, and optimizing quality of life. We are ranked in the top 1% of all hospitals in the nation for Neurology and Neurosurgery, and Montefiore Einstein’s Burke Rehabilitation Hospital is ranked in the top one percent of all U.S. hospitals for rehabilitation, according to U.S. News & World Report. Additionally, we are recipients of the highest standard for rehabilitation medicine, a three-year designation granted by the Commission on Accreditation of Rehabilitation Facilities (CARF).
At the Forefront of Treatment Innovation
We treat patients with state-of-the-art neurorestorative and neurorehabilitation techniques and advanced technologies, including artificial intelligence (AI), human-computer interfaces, robotics, neural regeneration and associated plasticity paradigms. These strategies enhance the ability of nerve cells and neural networks to adapt, change and self-organize to promote the formation of new neural network connections to greatly advance the treatment and recovery of functional disabilities associated with nervous system injuries and other impairments/disorders to optimize and accelerate the recovery of sustainable function. Our internationally recognized team of experts includes neurologists, neurorehabilitation physiatry specialists, neurosurgeons, neuroradiologists, bioengineers, neuropsychiatrists, neuropsychologists, speech-language pathologists, social workers and occupational, cognitive, and physical therapists who offer outpatient and inpatient neurorehabilitation and neurorestorative treatments.

Advanced Treatments
We use the latest and emerging diagnostics—including multimodal functional neuroimaging, sophisticated electrophysiological analyses and complementary molecular paradigms—to provide precision medicine approaches that promote a detailed understanding of the mechanisms that govern neuroplasticity, neuronal regeneration, resilience and recovery from disorders of the nervous system.
We are also at the forefront of a broad spectrum of research and technological innovations in restorative neurology. Our internationally renowned experts devise patient-specific plans for complex conditions utilizing the latest and most advanced neurobiologically informed treatments involving regenerative, neuromodulatory and/or enhanced neurorehabilitation strategies. Through team-based, interdisciplinary strategies—including early detection, targeted interventions, focused neuro-rehabilitation and complementary medicine approaches—we optimize functional outcomes, enhancing quality of life for patients and their families.
Some of the services we offer include:
Electrodiagnostics
Electromyography (EMG)
Electroencephalogram (EEG)
Nerve-conduction studies
Functional neuroimaging
Functional magnetic resonance imaging (fMRI)
Positron emission tomography (PET)
Neuropsychological evaluations
Pharmacologic interventions
Physical therapy
Occupational therapy
Vestibular therapy
Speech and language therapy
Cognitive rehabilitation
Rehabilitation psychology
Neuromodulation
Botulinum toxin (Botox) injections
State-of-the-Art Treatment Technologies
Our patients are treated with the latest neurorestorative and neurorehabilitation techniques and advanced technologies, including AI, human-computer interfaces, robotics, neural regeneration and associated plasticity paradigms.
Our team of experts utilizes the most advanced and newly emerging treatments, including stem cell therapy, neuromodulation such as spinal stimulation after spinal cord injury, AI and brain-computer interfaces, virtual reality and robotics (i.e., for stroke therapy), to not only restore function but to help modify the course of our patient’s disease.
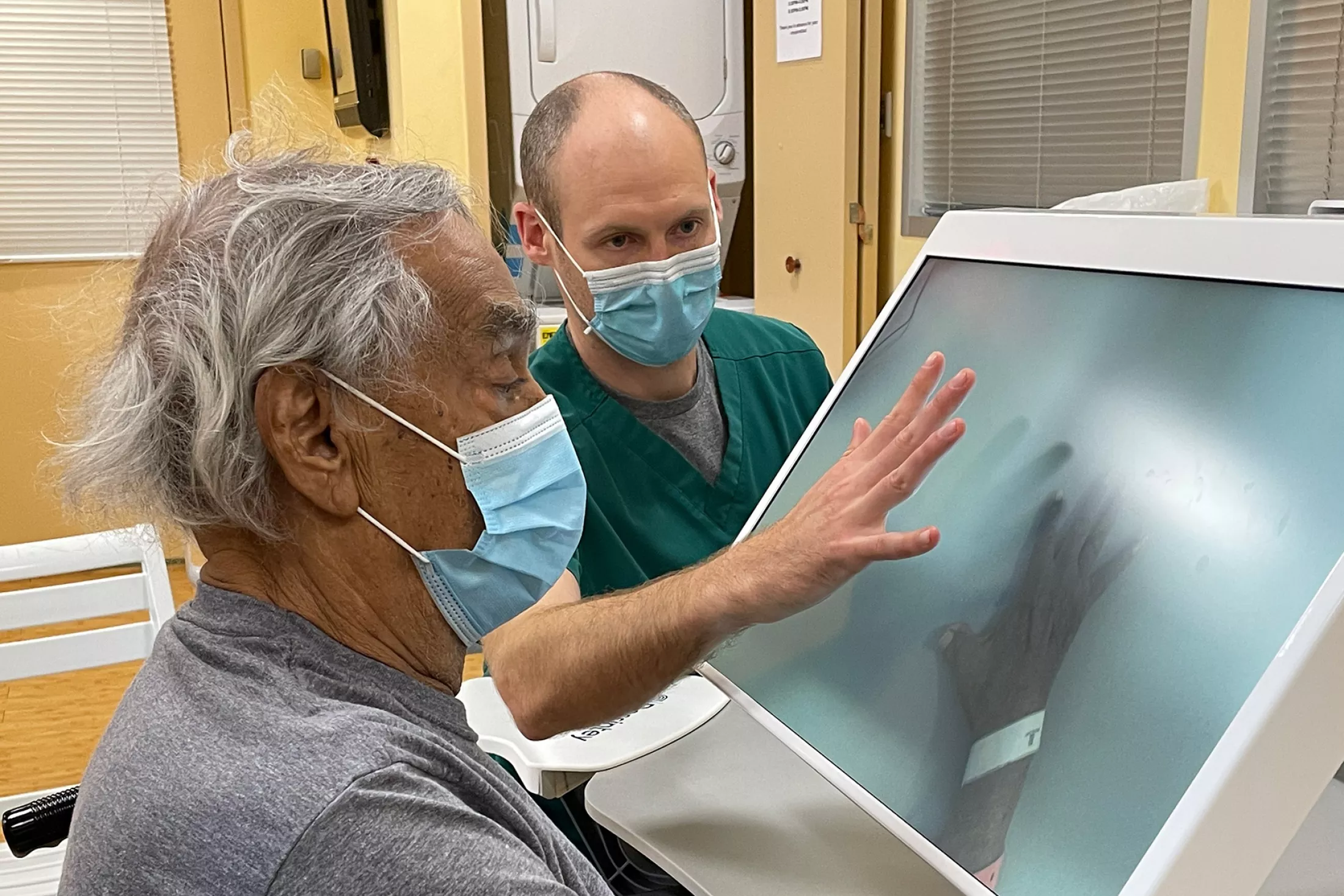
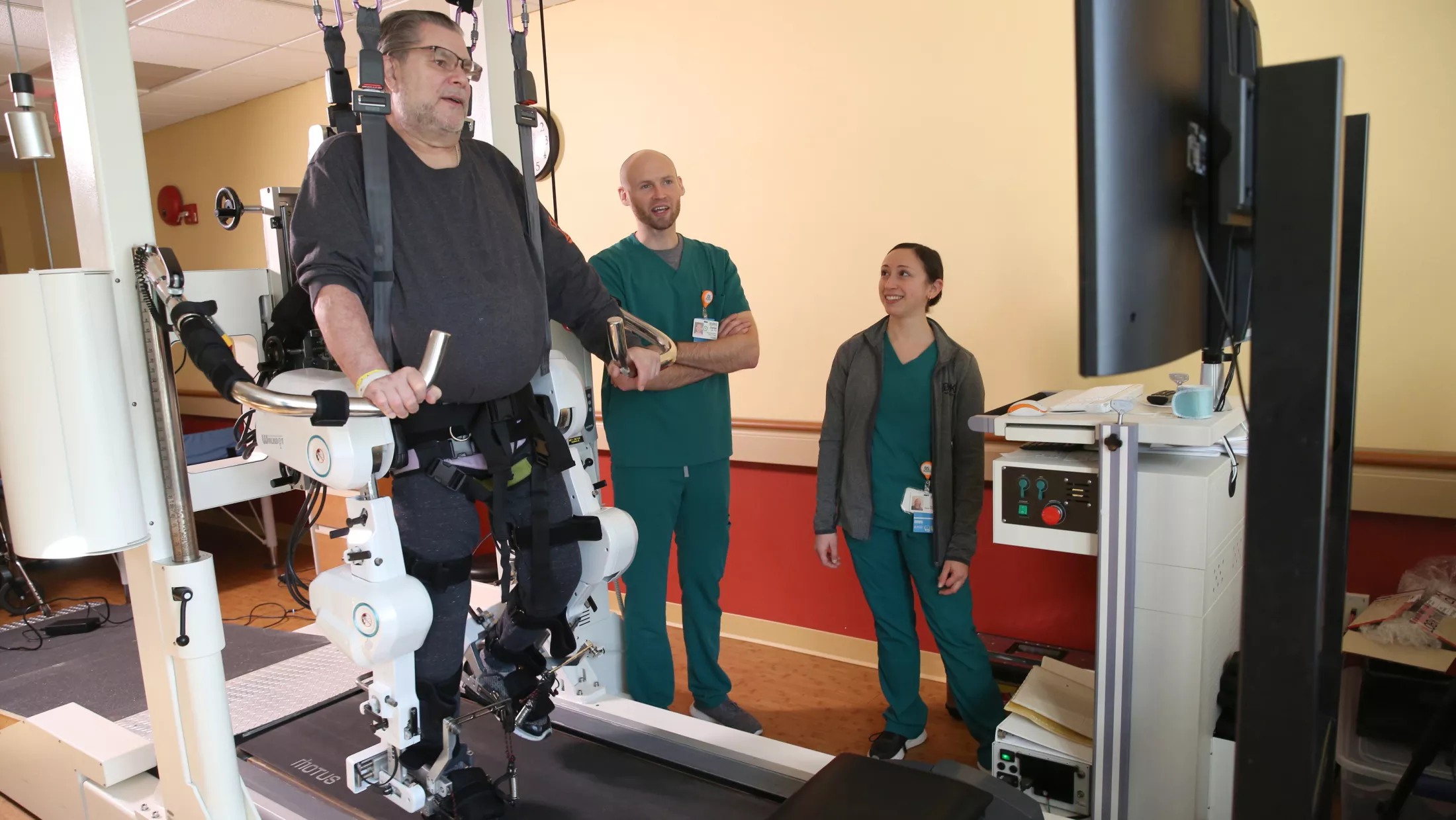
About Some of the Nervous System Disorders We Treat
Below are some of the nervous system disorders that we address at the Montefiore Einstein Restorative Neurology program.
Stroke
A stroke is often referred to as a “brain attack." It is a sudden interruption of continuous blood flow to the brain and is considered a medical emergency. A stroke occurs when a blood vessel in the brain becomes blocked or narrowed, or when a blood vessel bursts and spills blood into the brain. Just like a heart attack, a stroke requires immediate medical attention.
Multiple Sclerosis
Multiple sclerosis (MS) is the most common disabling neurological disease of young adults, with symptom onset generally occurring between the ages of 20 and 40 years. It is also the most common of the inflammatory demyelinating disorders, conditions wherein the immune system attacks the cells that produce and maintain the myelin sheath — a whitish protective coating over nerves that helps with electrical nerve signaling.
Parkinson’s Disease
Parkinson's disease (PD) is movement disorder of the nervous system that gets worse over time. As nerve cells (neurons) in parts of the brain weaken, are damaged, or die, people may begin to notice problems with movement, tremor, stiffness in the limbs or the trunk of the body, or impaired balance. As symptoms progress, people may have difficulty walking, talking or completing other simple tasks. Not everyone with one or more of these symptoms has PD, as the symptoms appear in other diseases as well.
Traumatic Brain Injury
A traumatic brain injury (TBI) can be caused by a forceful bump, blow or jolt to the head or body, or from an object that pierces the skull and enters the brain. Not all blows or jolts to the head result in a TBI. Primary effects on the brain include various types of bleeding and tearing forces that injure nerve fibers and cause inflammation, metabolic changes and brain swelling.
Spinal Cord Injury
A spinal cord injury (SCI) is damage to the tight bundle of cells and nerves that sends and receives signals from the brain to and from the rest of the body. The spinal cord extends from the lower part of the brain down through the lower back. A spinal cord injury can be classified by two types: complete or incomplete.