Cancer Dormancy Institute Service Core
Our team works closely with investigators, trainees and technical staff at Montefiore Einstein Comprehensive Cancer Center and Albert Einstein College of Medicine to advance cancer research on the biology of residual cancer cells that remain dormant before and after initial therapy, or as a consequence of therapy (persistent cancer cells).
We offer specialized services that address common needs for labs entering the exploration of cancer dormancy, including questions related to:
- What model to use
- Which markers to incorporate to profile cancer cells
- How to go about isolating these cancer cells
A major roadblock in studying cancer dormancy is choosing the right model for various cancer types. Dormant cells are rare and difficult to identify and characterize using standard methods. Specialized protocols, models and markers to detect and characterize these cells in the context of different cancer models are essential.
The Cancer Dormancy Institute’s Service Core provides investigators expert advice on markers, mouse models, protocol development and rare cell isolation methods. We can also assist in developing in vitro, murine and avian model studies related to metastasis, locoregional and distant disseminated cancer cell dormancy, and reactivation across several cancer models.
Our facility maintains a catalog of markers, validated antibodies and standardized protocols for cancer dormancy and recurrence studies. We provide advice and information on markers and methods to detect dormant cancer cells in human samples.
The Cancer Dormancy Institute’s Service Core additionally offers technical guidance on understanding how niche-specific environments can influence dormancy programming and reactivation. We can also assist in designing experimental approaches to answer specific questions.
This Service Core is designed to remove barriers to cancer dormancy research.
Director, Cancer Dormancy Institute
Contact Us for a Consultation
To support approved projects, Cancer Dormancy Institute’s Service Core Advisors Dr. Kadamb and Dr. Singh collaborate closely with leadership, offering guidance and advice to Cancer Center PI’s trainees, and technical staff undertaking the work. They can also coordinate the presentation of proposed projects to the team at the outset.
Cancer Dormancy Institute Service Core Advisors
Rama Kadamb, PhD
Staff Scientist
Email: rama.kadamb@einsteinmed.edu
Telephone: 718-678-1547
Deepak Singh, PhD
Instructor
Email: deepak.singh@einsteinmed.edu
Telephone: 718-678-1109
Technical & Advisory Services
Markers, Antibodies, Constructs & Detection Methods
We maintain an updated list of dormancy and reactivation markers and mediators that can be used in vitro, in vivo and in human sample studies, and we have validated antibodies and protocols for their detection across in vitro, in vivo and human samples. Senescence markers can be employed to distinguish dormant cancer cells. Other available methods include using DEPArrayTM and other rare cell isolation tools to isolate and profile circulating tumor cells (CTCs) or disseminated cancer cells in the lungs, brain, lymph nodes and bone marrow of mice, as well as isolating human CTCs.
Gene Signatures
We can provide access to gene signatures that inform on programs of dormancy in stem cells, cancer cells and embryonic stem cells, as well as signatures derived from senescent fibroblasts and other cancer models where dormancy was induced by pharmacological or genetic manipulation.
Small Molecules to Induce Dormancy or Target Dormant Cancer Cells
We provide information on commercially available small molecules, morphogens and biomolecules capable of inducing states of cancer cell dormancy and can advise on how to implement such pharmacological treatments.
Cell Lines
We maintain a repository of commonly used murine and human cell lines used in cancer dormancy and metastasis studies.
In Vitro Models
We provide technical help to establish 3D cultures, organoids, spheroids and cell tracing models that complement the in vivo models when studying dormancy mechanisms.
Syngeneic & Xenograft Mouse Models
We provide technical and experimental design guidance on how to use genetically engineered mouse models (GEMMs) for studies of dormancy and recurrence, as well as syngeneic models using mouse cell lines or primary cultures in vivo. We additionally offer technical and experimental design guidance for generating xenograft models from human cell lines or patient-derived xenografts provided by the investigator or supplied through our repository. Cell and tissue implantation methods include subcutaneous, intraperitoneal, intravenous, intracardiac, intrasplenic, intracranial and orthotopic. Mouse models also include GEMMs where specific reporters or perturbations affect host cells that are known to affect cancer cell dormancy. The latter allows exploring microenvironmental and niche-specific mechanisms dictating cancer cell dormancy. These models also include the analysis of immune and non-immune cell niches.
Fluorescent and/or Luciferase-Based & Cell Cycle-Based Reporters
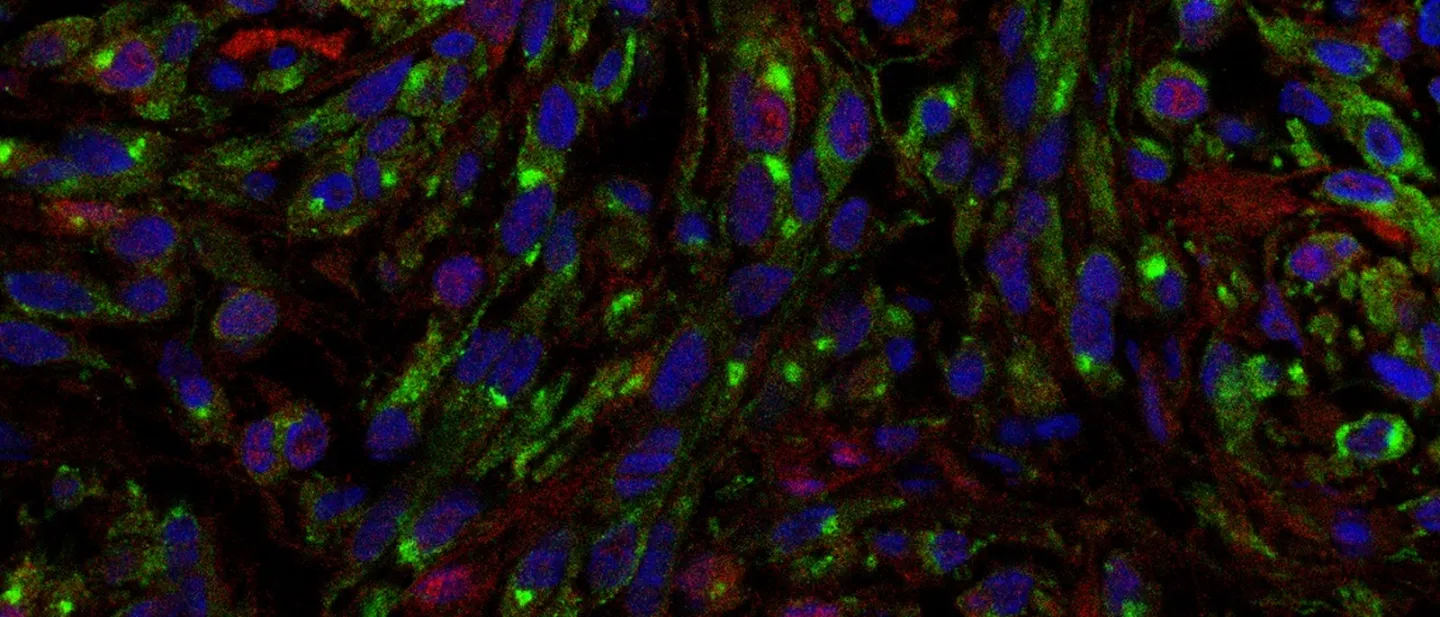
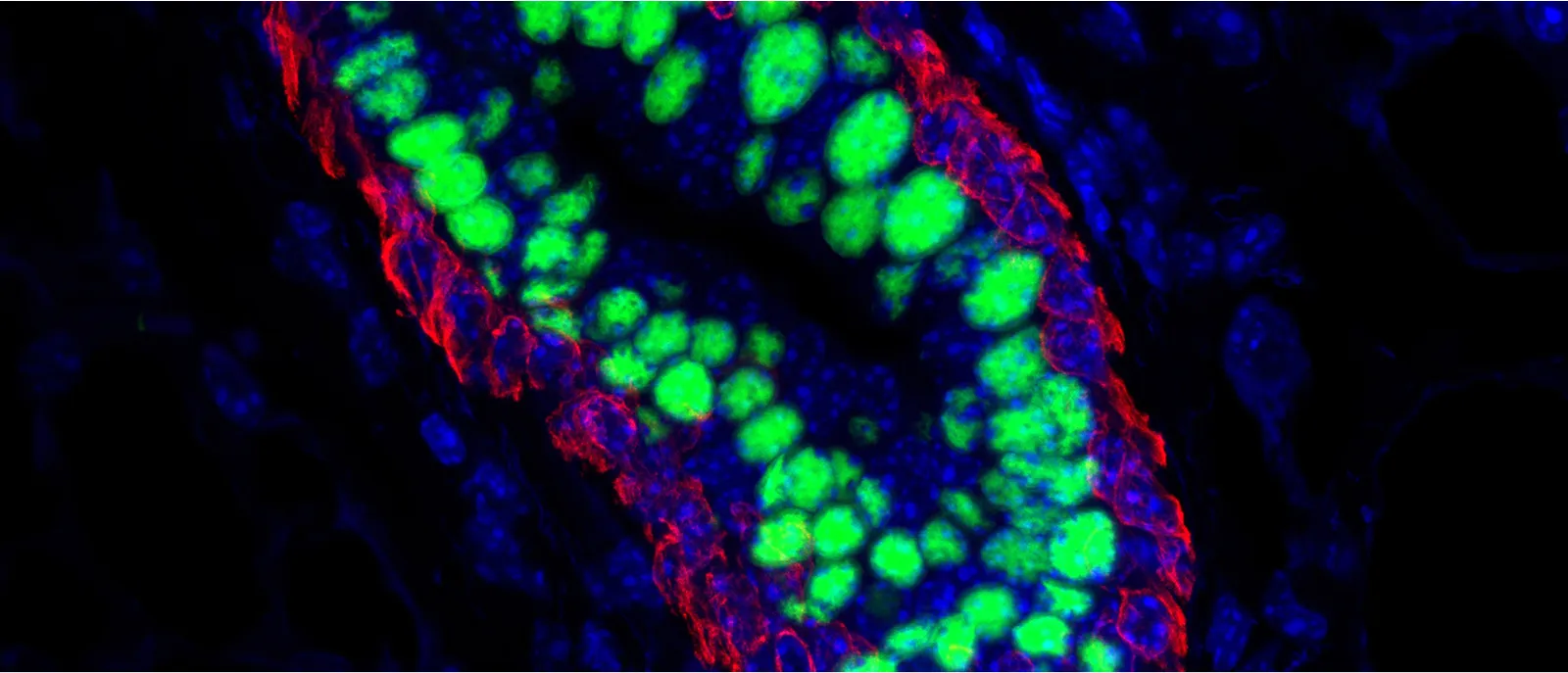
To identify dormant disseminated tumor cells in vitro and in vivo, we employ a toolbox of fluorescent and/or luciferase-based reporters and protein-barcoding tools. These tools are capable of monitoring in vitro and in vivo cancer dormancy, reactivation and clonal populations. Our lab has successfully used the following reporters and cell cycle-based sensors to identify dormant cells in vivo.
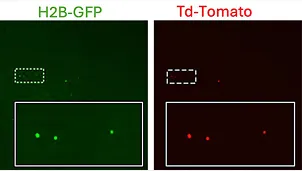
Dormant DTC’s
Proliferative Lesion
Detection of uveal melanoma dormant and proliferative DCCs in the mouse liver using H2B-GFP/td-Tomato label retention reporter system. Dormant UM DCCs express both H2B-GFP and td-Tomato, whereas proliferative UM lesion DCCs lose H2B-GFP expression and express only td-Tomato.
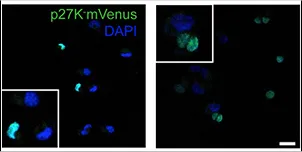
T-HEp3 cells with p27K-mVenus (mVenus signal indicates cell cycle arrest) bio-sensor.
Imaging Resources
In partnership with Albert Einstein College of Medicine’s Gruss Lipper Biophotonics Center, the Service Core can employ high-resolution microscopy of fixed or frozen tissues, as well as intravital imaging protocols, to give guidance on best practice protocols to detect and quantify dormant cancer cell phenotypes and the niches that harbor these cells.

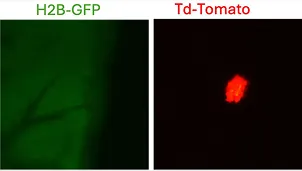
Interaction between alveolar macrophages and HER2+ early lesion DCCs in lung
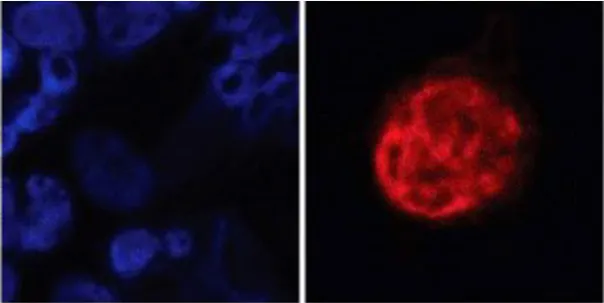
DCCs in the bone marrow of breast cancer patients
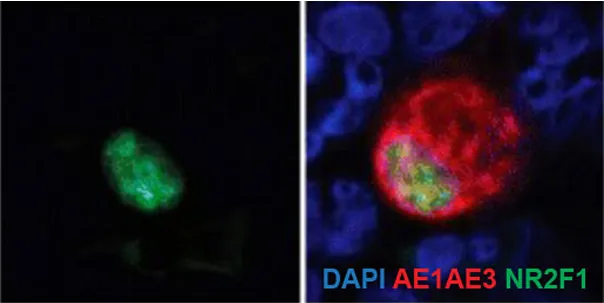
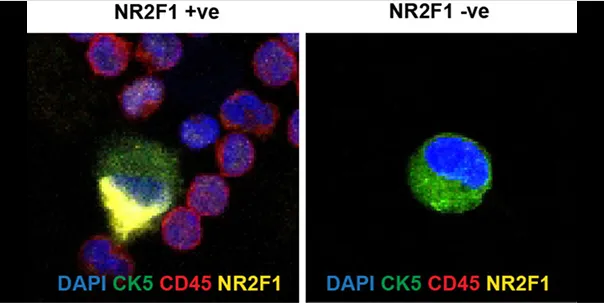
CTC detection in the blood of prostate cancer patient
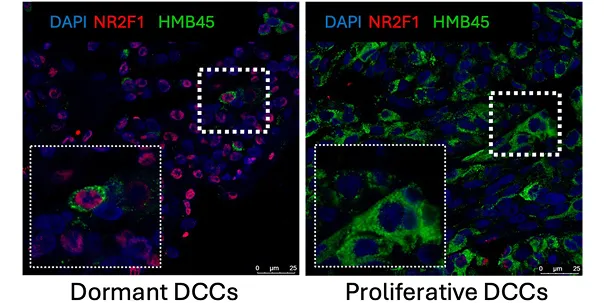
DCCs detection in the liver of uveal melanoma patient.
DCCs in the lungs of MMTV-HER2 mouse models
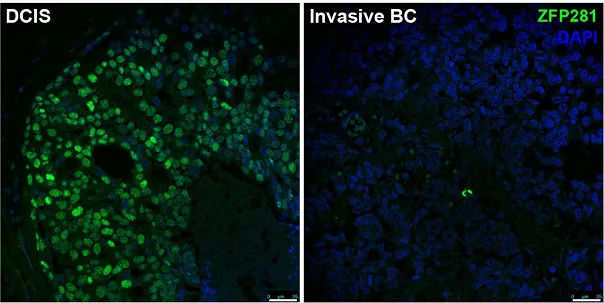
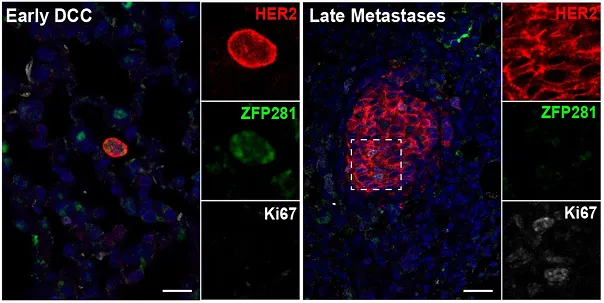
Dormancy marker (ZFP281) expression in breast cancer patients
Avian Chorioallantoic Membrane (CAM) Xenograft Model
We provide guidance and help to establish and experimental design for the CAM model. The CAM assay (Chorioallantoic Membrane Assay) is a widely used in vivo model for studying angiogenesis, tumor growth, metastasis, and drug testing. It utilizes the chorioallantoic membrane of a developing chicken embryo as a biological scaffold. Key features of the CAM model:
- Allows engraftment of human and mouse cancer cells without rejection due to the underdeveloped immune system of the chicken embryo.
- Useful to study cancer cell extravasation, a key step in metastasis, to different organs like the liver, lungs, and brain of developing embryos.
- Perfect model to monitor longitudinal tumor growth using non-invasive imaging methods using cells engineered to express fluorescent proteins such as H2B-GFP, p27-venus, and firefly luciferase.
Relevant Publications
Lung-resident Alveolar Macrophages Regulate the Timing of Breast Cancer Metastasis
Dalla E, Papanicolaou M, Park MD, Barth N, Hou R, Segura-Villalobos D, Valencia Salazar L, Sun D, Forrest ARR, Casanova-Acebes M, Entenberg D, Merad M, Aguirre-Ghiso JA. Cell. 2024 Nov 14;187(23):6631-6648.e20. doi: 10.1016/j.cell.2024.09.016. Epub 2024 Oct 7. PMID: 39378878.
MacroH2A Impedes Metastatic Growth by Enforcing a Discrete Dormancy Program in Disseminated Cancer Cells
Sun D, Singh DK, Carcamo S, Filipescu D, Khalil B, Huang X, Miles BA, Westra W, Sproll KC, Hasson D, Bernstein E, Aguirre-Ghiso JA. Sci Adv. 2022 Dec 2;8(48):eabo0876. doi: 10.1126/sciadv.abo0876. Epub 2022 Dec 2. PMID: 36459552; PMCID: PMC10936054.
ZFP281 Drives a Mesenchymal-Like Dormancy Program in Early Disseminated Breast Cancer Cells That Prevents Metastatic Outgrowth in the Lung
Nobre AR, Dalla E, Yang J, Huang X, Wullkopf L, Risson E, Razghandi P, Anton ML, Zheng W, Seoane JA, Curtis C, Kenigsberg E, Wang J, Aguirre-Ghiso JA. Nat Cancer. 2022 Oct;3(10):1165-1180. doi: 10.1038/s43018-022-00424-8. Epub 2022 Sep 1. PMID: 36050483.
Bone Marrow NG2+/Nestin+ Mesenchymal Stem Cells Drive DTC Dormancy Via TGFβ2
Nobre AR, Risson E, Singh DK, Di Martino JS, Cheung JF, Wang J, Johnson J, Russnes HG, Bravo-Cordero JJ, Birbrair A, Naume B, Azhar M, Frenette PS, Aguirre-Ghiso JA. Nat Cancer. 2021 Mar;2(3):327-339. doi: 10.1038/s43018-021-00179-8. Epub 2021 Mar 11. PMID: 34993493; PMCID: PMC8730384.
Mechanism of Early Dissemination and Metastasis in Her2+ Mammary Cancer
Harper KL, Sosa MS, Entenberg D, Hosseini H, Cheung JF, Nobre R, Avivar-Valderas A, Nagi C, Girnius N, Davis RJ, Farias EF, Condeelis J, Klein CA, Aguirre-Ghiso JA. Nature. 2016 Dec 22;540(7634):588-592. doi: 10.1038/nature20609. Epub 2016 Dec 14. Erratum in: Nature. 2018 Jan 18;553(7688):366. doi: 10.1038/nature24666. PMID: 27974798; PMCID: PMC5471138.
Dormancy in Breast Cancer
Erica Dalla # 1, Amulya Sreekumar # 2, Julio A Aguirre-Ghiso 3, Lewis A Chodosh 4 5
Mechanisms of Disseminated Cancer Cell Dormancy: An Awakening Field
María Soledad Sosa 1, Paloma Bragado 2, Julio A Aguirre-Ghiso 3
Models, Mechanisms and Clinical Evidence for Cancer Dormancy
Julio A Aguirre-Ghiso 1

Contact Us for a Consultation
Cancer Dormancy Institute Service Core Advisors
Rama Kadamb, PhD
Staff Scientist
Email: rama.kadamb@einsteinmed.edu
Telephone: 718-678-1547
Deepak Singh, PhD
Instructor
Email: deepak.singh@einsteinmed.edu
Telephone: 718-678-1109