Endometrial Cancer Screening
Finding cancer in its earliest stage offers the best chance of successful treatments. That’s why Montefiore Einstein Comprehensive Cancer Center is passionate about screenings for endometrial cancer and many other cancer types at our NCI-designated Comprehensive Cancer Center.
We are committed to finding endometrial cancer as early as possible, even before symptoms happen, so you can take steps to protect your health. Screening for these cancers is an important step in early identification.
At Montefiore Einstein Comprehensive Cancer Center, you can expect our compassionate and skilled healthcare providers to offer personalized screening in a supportive setting. And you’ll benefit from the latest, most advanced technology available to treat your cancer.
For more than 50 years, Montefiore Einstein Comprehensive Cancer Center has been a leader in the research, diagnosis and treatment of over 200 types of cancer. Turn to us for comprehensive cancer screening.

Cancer Clinical Trials
- Blood & Bone Marrow Cancers
- Brain, Spine & Central Nervous System Cancers
- Breast Cancer
- Childhood Cancers
- Endocrine System Cancers
- Gastrointestinal (GI) Cancers
- Genitourinary (GU) & Urologic Cancers
- Gynecologic Cancers
- Head & Neck Cancers
- Kaposi Sarcoma & AIDS-Related Cancers
- Lung & Chest Cancers
- Prostate Cancer
- Sarcomas
- Skin Cancer
As an NCI-designated comprehensive cancer center, Montefiore Einstein Comprehensive Cancer Center supports the mission and guidelines of the National Cancer Institute (NCI). The following information about types of cancer, prevention and treatments is provided by the NCI.
Endometrial Cancer Screening (PDQ®)–Patient Version
What Is Screening?
Screening is looking for cancer before a person has any symptoms. This can help find cancer at an early stage. When abnormal tissue or cancer is found early, it may be easier to treat. By the time symptoms appear, cancer may have begun to spread.
Scientists are trying to better understand which people are more likely to get certain types of cancer. They also study the things we do and the things around us to see if they cause cancer. This information helps doctors recommend who should be screened for cancer, which screening tests should be used, and how often the tests should be done.
It is important to remember that your doctor does not necessarily think you have cancer if he or she suggests a screening test. Screening tests are given when you have no cancer symptoms.
If a screening test result is abnormal, you may need to have more tests done to find out if you have cancer. These are called diagnostic tests.
General Information About Endometrial Cancer
Key Points
- Endometrial cancer is a disease in which malignant (cancer) cells form in the tissues of the endometrium.
- Endometrial cancer is most common in postmenopausal women.
- Different factors increase or decrease the risk of getting endometrial cancer.
Endometrial cancer is a disease in which malignant (cancer) cells form in the tissues of the endometrium.
The endometrium is the innermost lining of the uterus. The uterus is a hollow, muscular organ in a woman's pelvis. The uterus is where a fetus grows. In most nonpregnant women, the uterus is about 3 inches long.
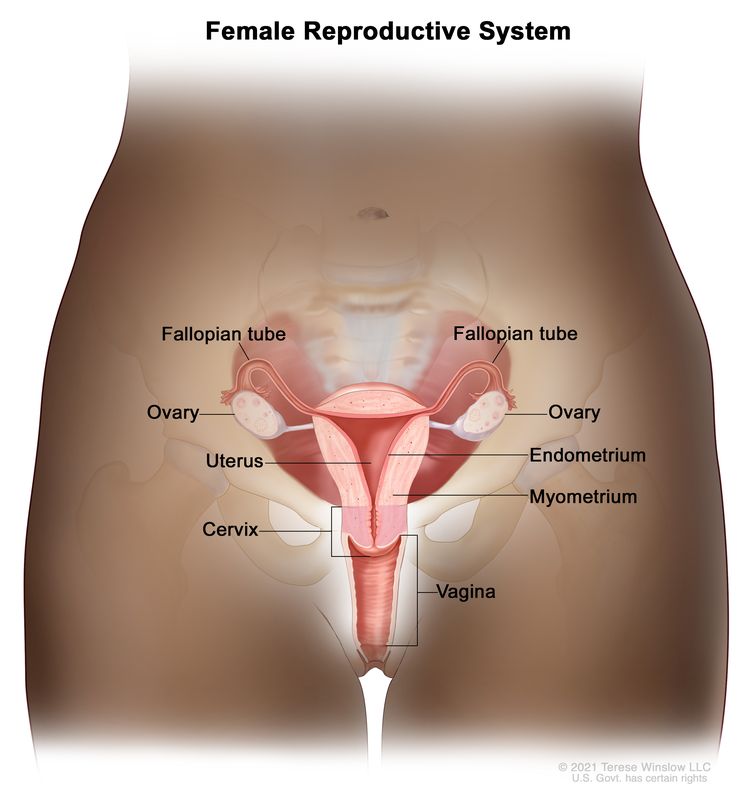
Anatomy of the female reproductive system. The organs in the female reproductive system include the uterus, ovaries, fallopian tubes, cervix, and vagina. The uterus has a muscular outer layer called the myometrium and an inner lining called the endometrium.
Cancer of the endometrium is different from cancer of the muscle of the uterus, which is called uterine sarcoma. For more information, visit Uterine Sarcoma Treatment.
Other PDQ summaries containing information related to endometrial cancer include:
Endometrial cancer is most common in postmenopausal women.
Endometrial cancer occurs most often in postmenopausal women, with 60 being the average age at diagnosis.
From 2012 to 2021, the number of new cases of endometrial cancer increased slightly in White women and by 2% to 3% each year in women of all other racial and ethnic groups. From 2013 to 2022, the number of deaths from endometrial cancer increased by just under 2% each year.
Different factors increase or decrease the risk of getting endometrial cancer.
Anything that increases your chance of getting a disease is called a risk factor. Anything that decreases your chance of getting a disease is called a protective factor.
Learn more about risk factors and protective factors for endometrial cancer in Endometrial Cancer Prevention.
Endometrial Cancer Screening
Key Points
- Tests are used to screen for different types of cancer when a person does not have symptoms.
- Endometrial cancer is usually found early.
- There is no standard or routine screening test for endometrial cancer.
- Tests that may detect (find) endometrial cancer are being studied:
- Pap test
- Transvaginal ultrasound
- Endometrial sampling
- Screening tests for endometrial cancer are being studied in clinical trials.
Tests are used to screen for different types of cancer when a person does not have symptoms.
Scientists study screening tests to find those with the fewest harms and most benefits. Cancer screening trials also are meant to show whether early detection (finding cancer before it causes symptoms) helps a person live longer or decreases a person's chance of dying from the disease. For some types of cancer, the chance of recovery is better if the disease is found and treated at an early stage.
Endometrial cancer is usually found early.
Endometrial cancer usually causes symptoms (such as vaginal bleeding) and is found at an early stage, when there is a good chance of recovery.
There is no standard or routine screening test for endometrial cancer.
Screening for endometrial cancer is under study and there are screening clinical trials taking place in many parts of the country. Information about ongoing clinical trials is available from the NCI website.
Tests that may detect (find) endometrial cancer are being studied:
Pap test
A Pap test is a procedure to collect cells from the surface of the cervix and vagina. A piece of cotton, a brush, or a small wooden stick is used to gently scrape cells from the cervix and vagina. The cells are viewed under a microscope to find out if they are abnormal. This procedure is also called a Pap smear.
Pap tests are not used to screen for endometrial cancer; however, Pap test results sometimes show signs of an abnormal endometrium (lining of the uterus). Follow-up tests may detect endometrial cancer.
Transvaginal ultrasound
No studies have shown that screening by transvaginal ultrasound (TVU) lowers the number of deaths caused by endometrial cancer.
Transvaginal ultrasound (TVU) is a procedure used to examine the vagina, uterus, fallopian tubes, and bladder. It is also called endovaginal ultrasound. An ultrasound transducer (probe) is inserted into the vagina and used to bounce high-energy sound waves (ultrasound) off internal tissues or organs and make echoes. The echoes form a picture of body tissues called a sonogram. The doctor can identify tumors by looking at the sonogram.
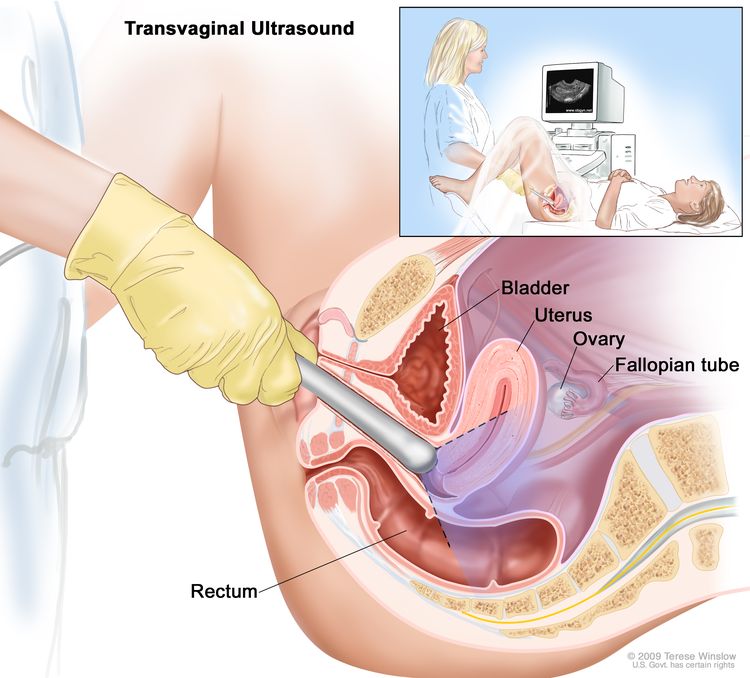
Transvaginal ultrasound. An ultrasound probe connected to a computer is inserted into the vagina and is gently moved to show different organs. The probe bounces sound waves off internal organs and tissues to make echoes that form a sonogram (computer picture).
TVU is commonly used to examine women who have abnormal vaginal bleeding. For women who have or are at risk for hereditary nonpolyposis colon cancer, experts suggest yearly screening with transvaginal ultrasound, beginning as early as age 25.
The use of tamoxifen to treat or prevent breast cancer increases the risk of endometrial cancer. TVU is not useful in screening for endometrial cancer in women who take tamoxifen but do not have any symptoms of endometrial cancer. In women taking tamoxifen, TVU should be used in those who have vaginal bleeding.
Endometrial sampling
It has not been proven that screening by endometrial sampling (biopsy) lowers the number of deaths caused by endometrial cancer.
Endometrial sampling is the removal of tissue from the endometrium by inserting a brush, curette, or thin, flexible tube through the cervix and into the uterus. The tool is used to gently scrape a small amount of tissue from the endometrium and then remove the tissue samples. A pathologist views the tissue under a microscope to look for cancer cells.
Endometrial sampling is commonly used to examine women who have abnormal vaginal bleeding. If you have abnormal vaginal bleeding, check with your doctor.
Screening tests for endometrial cancer are being studied in clinical trials.
Information about clinical trials supported by NCI can be found on NCI’s clinical trials search webpage. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
Risks of Endometrial Cancer Screening
Key Points
- Screening tests have risks.
- The risks of endometrial cancer screening tests include:
- Finding endometrial cancer may not improve health or help a woman live longer.
- False-negative test results can occur.
- False-positive test results can occur.
- Side effects may be caused by the test itself.
Screening tests have risks.
Decisions about screening tests can be difficult. Not all screening tests are helpful and most have risks. Before having any screening test, you may want to discuss the test with your doctor. It is important to know the risks of the test and whether it has been proven to reduce the risk of dying from cancer.
The risks of endometrial cancer screening tests include:
Finding endometrial cancer may not improve health or help a woman live longer.
Screening may not improve your health or help you live longer if you have advanced endometrial cancer or if it has already spread to other places in your body.
Some cancers never cause symptoms or become life-threatening, but if found by a screening test, the cancer may be treated. It is not known if treatment of these cancers would help you live longer than if no treatment were given, and treatments for cancer may have serious side effects.
False-negative test results can occur.
Screening test results may appear to be normal even though endometrial cancer is present. A woman who receives a false-negative test result (one that shows there is no cancer when there really is) may delay seeking medical care even if she has symptoms.
False-positive test results can occur.
Screening test results may appear to be abnormal even though no cancer is present. A false-positive test result (one that shows there is cancer when there really isn't) can cause anxiety and is usually followed by more tests (such as biopsy), which also have risks.
Side effects may be caused by the test itself.
Side effects that may be caused by screening tests for endometrial cancer include:
If you have any questions about your risk for endometrial cancer or the need for screening tests, check with your doctor.
About This PDQ Summary
About PDQ
Physician Data Query (PDQ) is the National Cancer Institute's (NCI's) comprehensive cancer information database. The PDQ database contains summaries of the latest published information on cancer prevention, detection, genetics, treatment, supportive care, and complementary and alternative medicine. Most summaries come in two versions. The health professional versions have detailed information written in technical language. The patient versions are written in easy-to-understand, nontechnical language. Both versions have cancer information that is accurate and up to date and most versions are also available in Spanish.
PDQ is a service of the NCI. The NCI is part of the National Institutes of Health (NIH). NIH is the federal government’s center of biomedical research. The PDQ summaries are based on an independent review of the medical literature. They are not policy statements of the NCI or the NIH.
Purpose of This Summary
This PDQ cancer information summary has current information about endometrial cancer screening. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Reviewers and Updates
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Updated") is the date of the most recent change.
The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Screening and Prevention Editorial Board.
Clinical Trial Information
A clinical trial is a study to answer a scientific question, such as whether one treatment is better than another. Trials are based on past studies and what has been learned in the laboratory. Each trial answers certain scientific questions in order to find new and better ways to help cancer patients. During treatment clinical trials, information is collected about the effects of a new treatment and how well it works. If a clinical trial shows that a new treatment is better than one currently being used, the new treatment may become "standard." Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Clinical trials can be found online at NCI's website. For more information, call the Cancer Information Service (CIS), NCI's contact center, at 1-800-4-CANCER (1-800-422-6237).
Permission to Use This Summary
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks in the following way: [include excerpt from the summary].”
The best way to cite this PDQ summary is:
PDQ® Screening and Prevention Editorial Board. PDQ Endometrial Cancer Screening. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/uterine/patient/endometrial-screening-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389486]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online. Visuals Online is a collection of more than 3,000 scientific images.
Disclaimer
The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s E-mail Us.
Updated:
Source URL: https://www.cancer.gov/node/4885/syndication
Source Agency: National Cancer Institute (NCI)
Captured Date: 2013-09-14 09:02:29.0