We are among the elite 1% NCI-designated comprehensive cancer centers in the U.S. — and we are ranked in the top 1% of all U.S. hospitals for cancer care, according to U.S. News & World Report.


Cancer Clinical Trials
- Blood & Bone Marrow Cancers
- Brain, Spine & Central Nervous System Cancers
- Breast Cancer
- Childhood Cancers
- Endocrine System Cancers
- Gastrointestinal (GI) Cancers
- Genitourinary (GU) & Urologic Cancers
- Gynecologic Cancers
- Head & Neck Cancers
- Kaposi Sarcoma & AIDS-Related Cancers
- Lung & Chest Cancers
- Prostate Cancer
- Sarcomas
- Skin Cancer
Treating More Than 200 Types of Cancer
Over 300 Active Clinical Trials
Navigating Your Cancer Care
At Montefiore Einstein Comprehensive Cancer Center, you receive evidence-based cancer care that addresses the unique needs of each individual patient. Our NCI-designated comprehensive cancer center brings the worlds of clinical care and academic research together and allows for the translation of novel, leading-edge scientific discoveries into improved clinical outcomes and new life-saving treatments.
Using a multidisciplinary team of disease-focused experts, our full-service cancer center is focused on the prevention, early diagnosis and treatment of cancer while incorporating a comprehensive spectrum of traditional and holistic care. This allows us to deliver highly specialized and coordinated compassionate care for the whole person, address complex medical needs and improve the quality of life for each and every patient.

Over 50 Years of Cancer Research & Treatment Breakthroughs

Our Latest News & Research
Advancing human health, scientific discovery and innovation through cancer research
Leaders in the Science of Cancer Care
At Montefiore Einstein Comprehensive Cancer Center, we’re committed to finding new and better cancer treatment options.
Our research breakthroughs continue to lead to game-changing cancer treatment practices and give patients a chance to join upcoming clinical trials, which may offer potentially successful treatment options. We’ve received accreditation from the NCI for more than 50 years in a row. Our facility is one of only 56 NCI-designated comprehensive cancer centers across the United States, which means that through the NCI peer review process, we’ve been recognized by our national colleagues for the remarkable strengths of our exceptional science integrated with extraordinary multidisciplinary patient care. We’re also the only NCI-designated comprehensive cancer center located between Westchester and Buffalo. We made that expansion from Manhattan to Westchester to provide convenient access to cancer patients across the region.

State-of-the-Art Technology
At Montefiore Einstein Comprehensive Cancer Center, we are proud to provide patients with the newest technology available and offer the latest surgical and therapeutic techniques. Our goal is to utilize the least invasive solutions for your cancer care.
That is why we offer the latest in image-guided, immuno-, cellular and targeted therapies, to name just a few.
Some of our other molecular imaging technology includes the latest three-dimensional systems for bioluminescence imaging (BLI), fluorescence molecular imaging (FMI), positron emission tomography (PET) and single-photon emission computed tomography (SPECT).
Committed to Moving Faster Than Cancer
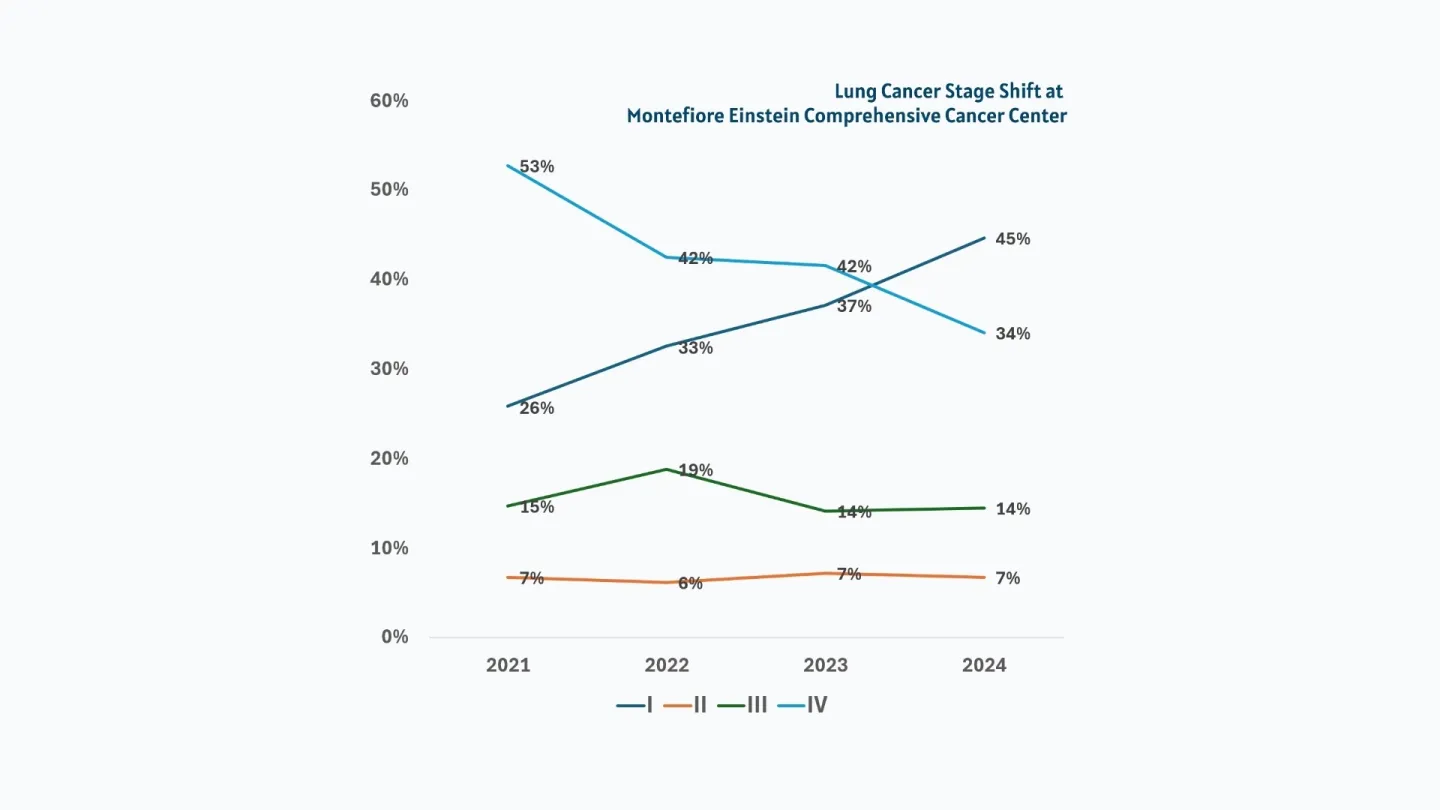
Cancer Center Highlights Report
Our Highlights Report documents our remarkable innovations in research, treatment and care.
-
$82M
in cancer relevant funding, of which $33 million is dedicated to philanthropy
-
1000+
patients enrolled in clinical research studies
-
400
papers published annually in high impact peer-reviewed journals
-
400+
types of cancer treated
Meet Your Cancer Care Team
Montefiore Einstein Comprehensive Cancer Center is home to 14 teams of award-winning, multidisciplinary experts who will develop a highly personalized treatment plan specific to your particular type of cancer, including those considered rare.
Events at Montefiore Einstein Comprehensive Cancer Center
At Montefiore Einstein Comprehensive Cancer Center, we’re passionate about enriching the health and well-being of our community. To meet different interests and needs, we offer in-person and virtual events, classes, support groups and other activities year-round.
Study of JANX007 in Subjects With Metastatic Castration-Resistant Prostate Cancer (ENGAGER-PSMA-01)
-
Start Date
September 15, 2022
-
Conditions
Prostate Cancer, Metastatic Castration-Resistant Prostate Cancer, Castration-Resistant Prostatic Cancer
A Study of Amivantamab and FOLFIRI Versus Cetuximab/Bevacizumab and FOLFIRI in Participants With KRAS/NRAS and BRAF Wild-type Colorectal Cancer Who Have Previously Received Chemotherapy
-
Start Date
December 12, 2024
-
Conditions
Colorectal Neoplasms
VO and Nivolumab vs Physician's Choice in Advanced Melanoma That Progressed on Anti-PD-1 & Anti-CTLA-4 Drugs (IGNYTE-3)
-
Start Date
July 11, 2024
-
Conditions
Advanced Melanoma
EF-41/KEYNOTE D58: Phase 3 Study of Optune Concomitant With Temozolomide Plus Pembrolizumab in Newly Diagnosed Glioblastoma
-
Start Date
February 3, 2025
-
Conditions
Glioblastoma
Alpha Radiation Emitters Device for the Treatment of Pancreatic Cancer Emitters With Chemotherapy for the Treatment of Locally Advanced and Metastatic Pancreatic Cancer
-
Start Date
June 17, 2025
-
Conditions
Pancreatic Cancer, Pancreatic Adenocarcinoma, Metastatic Pancreatic Cancer
Thoracic Radiotherapy and Inhibition of PD-1 and LAG-3 for Locally Advanced Non-Small Cell Lung Cancer
-
Start Date
August 7, 2025
-
Conditions
Non-Small-Cell Lung Cancer, Locally Advanced