Feature
Celebrating Pathology’s Role in Breast Cancer Diagnosis and Research
October 28, 2024

Members of the Breast Pathology Service and pathology researchers investigating metastasis
October is Breast Cancer Awareness Month, and this year, we celebrate the Pathology Department's outstanding team of surgical breast pathologists and the clinical, basic science, and translational researchers, members of the Montefiore Einstein Comprehensive Cancer Center, studying breast cancer metastasis. These expert clinicians and researchers play a critical role in diagnosing and treating breast cancer while driving groundbreaking research that deepens our understanding of metastasis and helps develop better, more effective therapies.
Surgical Breast Pathology Service
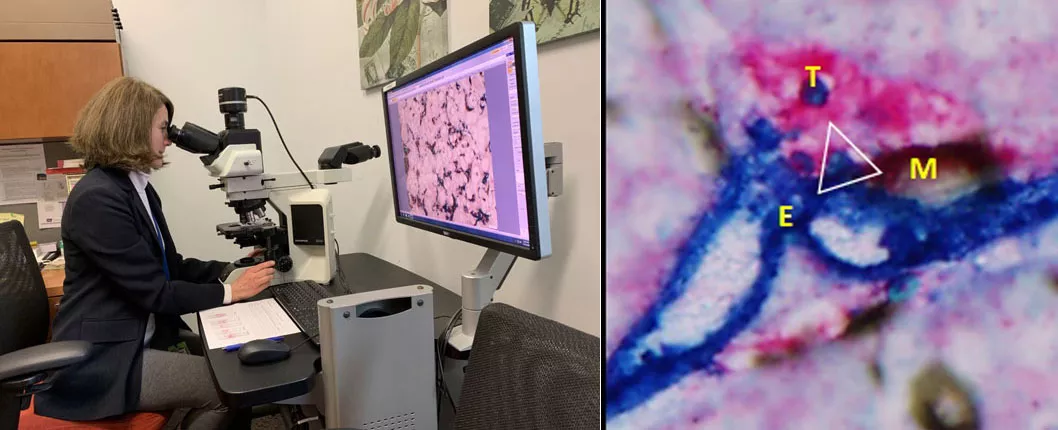
Led by section head Susan Fineberg, MD, the Breast Pathology Service at Montefiore consists of eight highly trained surgical anatomic pathologists: Kathleen Whitney, MD (director of surgical pathology), Jennifer Oliver-Krasinski, MD (pediatric and breast pathologist), Berrin Ustun, MD, Javier Laurini, MD, Bryan Harmon, MD, Sonali Lanjiwar, MD, Rouzan Karabakhstian, MD, PhD (director of the Women's Health Pathology Fellowship program), and Dr. Fineberg, a dedicated breast pathologist. As critical members of the patient care team, the pathologists analyze breast tissue from tumor biopsies, lumpectomies, mastectomies, and other procedures to determine diagnoses that guide treatment plans for Montefiore patients. In addition to breast cases, these experts also diagnose various women's health and anatomical pathology cases.
‘We’re Only as Good as our Pathologists’
Breast oncologist Delia Makower, MD, an associate professor of medicine and medical oncology and the director of Therapeutic Services for Breast Medical Oncology at Montefiore Einstein, works closely with Dr. Fineberg and the surgical pathology breast team. She emphasizes pathologists' critical role in diagnosing breast disease and collaborating with oncologists and breast surgeons to develop patient treatment plans.
"We are only as good as our pathologists – we rely on them completely," Dr. Makower said, highlighting their essential role in diagnosing tumors, identifying biomarkers, and serving as integral members of the multidisciplinary tumor board. "The diagnosis they provide determines the treatment we offer our breast cancer patients."
A Complex Caseload
The breast pathology service at MMC processes the tissue from roughly 2,400 new breast cases each year. Each case requires the microscopic examination of diagnostic slides, with many requiring more sophisticated biomarker or genomic testing performed and analyzed by our pathologists. "We take into account both clinical and imaging findings, often coupled with consultation with our clinical colleagues to arrive at a precise diagnosis," explained Dr. Fineberg.
Overall, the most common type of breast cancer is estrogen receptor-positive or hormone receptor-positive breast cancer. Montefiore encounters a higher percentage of advanced-stage cancers, particularly triple-negative breast cancers (TNBC), which is the most aggressive subtype. Promisingly, immunotherapy is emerging as a viable treatment option for TNBC. Additionally, Montefiore's caseload includes a higher proportion of younger breast cancer patients compared to surrounding institutions.
Guardians of Tissue for Clinical Research and Collaborations
The pathology department stores the breast tissue samples and makes them available for researchers at Montefiore and Einstein for IRB-approved investigations. As keepers of the tissue, pathologists play a crucial role in supporting clinical trials and research collaborations, says Dr. Kathleen Whitney, director of surgical pathology at Montefiore Einstein. This access allows for exploring molecular and genetic markers, driving discoveries of novel biomarkers and therapeutic targets.
Dr. Fineberg's research focuses on advancing biomarker development in invasive breast cancer, identifying prognostic markers for ductal carcinoma in situ (DCIS), clarifying the role of immune responses in breast cancer progression, and standardizing pathology practices to ensure consistency in breast cancer diagnosis and research.
Among her work is a collaboration with Wenjun Guo, PhD, from the Department of Cell Biology, where they examine the molecular mechanisms underlying aggressive basal-like breast cancer. In addition, she partners with researchers at Oregon Health & Science University. She and her team will present insights from this research at the upcoming USCAP meeting in Boston, scheduled for March 22-27, 2025.
Beyond these projects, Dr. Fineberg is actively involved in a multicenter study examining EZH2 as a predictive biomarker for endocrine therapy response in hormone receptor-positive cancers. She collaborates with a multidisciplinary team at Montefiore, led by Tim Duong, PhD, from the departments of radiology and cell biology, to investigate the potential of combining AI with advanced breast imaging techniques for predicting tumor characteristics and treatment response. Together, these studies are part of a broader effort to personalize breast cancer treatment and improve outcomes for patients across diverse backgrounds.
Pioneering Work in Biomarker Reporting and Personalized Medicine
The complex caseload at Montefiore underscores the importance of identifying targetable biomarkers and establishing standardized reporting approaches, allowing for more personalized treatment strategies based on tumor characteristics.
Collaborating with the International Immuno-Oncology Biomarker Working Group, Dr. Fineberg has contributed significantly to developing guidelines for two key biomarkers: Ki67 and Tumor Infiltrating Lymphocytes (TILs).
- Ki67: This protein marker indicates tumor growth aggressiveness and is crucial for determining prognosis and guiding therapy for hormone receptor-positive breast cancers.
- TILs: The presence of immune cells within tumors correlates with better prognoses in TNBC. Reporting on TILs helps predict how cancers might respond to chemotherapy.
Investigating Breast Cancer Metastasis
Department of Pathology researchers Rachel Hazan, PhD, Maja Oktay, MD, PhD, and David Entenberg, PhD, are advancing our understanding of breast cancer metastasis and exploring improved treatment methods. The three researchers are members of the Montefiore Einstein Comprehensive Cancer Center (MECCC) and are part of a NIH-funded Program Project Grant (PPG), led by Jonathan Backer, MD, professor of molecular pharmacology and associate director of Shared Resources at MECCC, focusing on metastasis mechanisms.
Rachel Hazan, PhD: Mechanisms of Metastasis
Dr. Rachel Hazan, a professor of pathology at Einstein, investigates the cellular changes driving breast cancer metastasis and the relationship between cell-cell adhesion, receptor tyrosine kinases, and redox signaling in driving epithelial to mesenchymal transitions and cancer stem cells. A $225,000 grant from the Breast Cancer Research Foundation supports Dr. Hazan's research.
In a study published in January 2024 in the British Journal of Cancer, Dr Hazan and colleagues show the role of GPx2 loss in promoting epithelial-to-mesenchymal transition (EMT), a process crucial for metastasis. Using single-cell RNA sequencing, they show how different tumor cell clusters evolve and exhibit varying degrees of EMT, including hybrid EMT states, which enhance metastatic potential. This study focuses on phenotypic plasticity and highlights the role of p63 activation in these cellular transitions, suggesting that targeting this pathway could suppress metastasis.
In an earlier study, Dr. Hazan and colleagues examined how the loss of GPx2 increases reactive oxygen species (ROS), activating the HIF1α pathway, which leads to metabolic changes within the tumor. It highlights the tumor's metabolic plasticity, where some cells adapt to hypoxic conditions while others exhibit a hybrid state, using both glycolysis and oxidative phosphorylation. This flexibility allows tumor cells to survive under varying conditions, and the paper suggests that targeting these metabolic states could be a promising therapeutic strategy. The findings were published in February 2022 in PNAS. Dr. Hazan presented her findings at the recent MECCC retreat, stressing the need for new therapeutic strategies.
Real-Time Visualization of Metastasis
Utilizing intravital imaging to observe cancer cell migration, Drs. Maja Oktay and David Entenberg investigate how breast cancer cells interact with the body's environment. Their research aims to use the knowledge gained from these imaging studies to create therapies that interrupt these interactions and to create biomarkers that aid in patient care.
Drs. Oktay, Entenberg, and colleague John Condeelis, PhD, the Judith and Burton P. Resnick Chair in Translational Research, Scientific Director, Analytical Imaging Facility, and co-director of the Integrated Imaging Program for Cancer Research, are funded by a multi-PI National Institutes of Health National Cancer Institute (NCI) R01 grant and an NCI-funded Program Project Grant led by Jonathan Backer, MD, professor of molecular pharmacology and associate director of Shared Resources at MACE, focusing on metastasis mechanisms. The Gruss Lipper Charitable Foundation within the Integrated Imaging Program for Cancer Research (IIPCR) also funds their research.
Maja Oktay, MD. PhD: Physician-Scientist focused on TMEM Doorways
Dr. Maja Oktay, a professor of pathology, and attending cytopathologist and cancer cell biology researcher, focuses on the Tumor Micro-Environment of Metastasis (TMEM) doorways, demonstrating their role in cancer cell migration and metastasis. Her studies highlight TMEM doorway score as a prognostic marker of metastasis and a site where cancer cells get educated to become stem and invasive. In addition, her work revealed that chemotherapy-induced changes can be reversed with TIE2 inhibition. This work formed a basis for a phase 1b clinical of TIE2 inhibitor rebastinib plus chemotherapy (paclitaxel or eribulin) in HER2-negative metastatic breast cancer led by Jesus D. Anampa Mesias, M.D., M.S. and co-authored by Dr. Oktay. The study identified an acceptable rebastinib dose range and pharmacodynamic evidence of TIE2 kinase inhibition providing a foundation for additional studies evaluating TIE2 kinase inhibitors in combination with other systemic therapies. This study was recently accepted for publication in Clinical Cancer Research.
As a physician-scientist, Dr. Oktay also investigates molecular biomarkers, and the impact of treatments on breast cancer progression.
Dr. Oktay co-leads the Tumor Microenvironment and Metastasis Program at MECCC, directs the New York Pathology Oncology Group, and co-directs the Integrated Imaging Program for Cancer Research (IIPCR) with John Condeelis, and leads the IIPCR Breast Cancer Program.
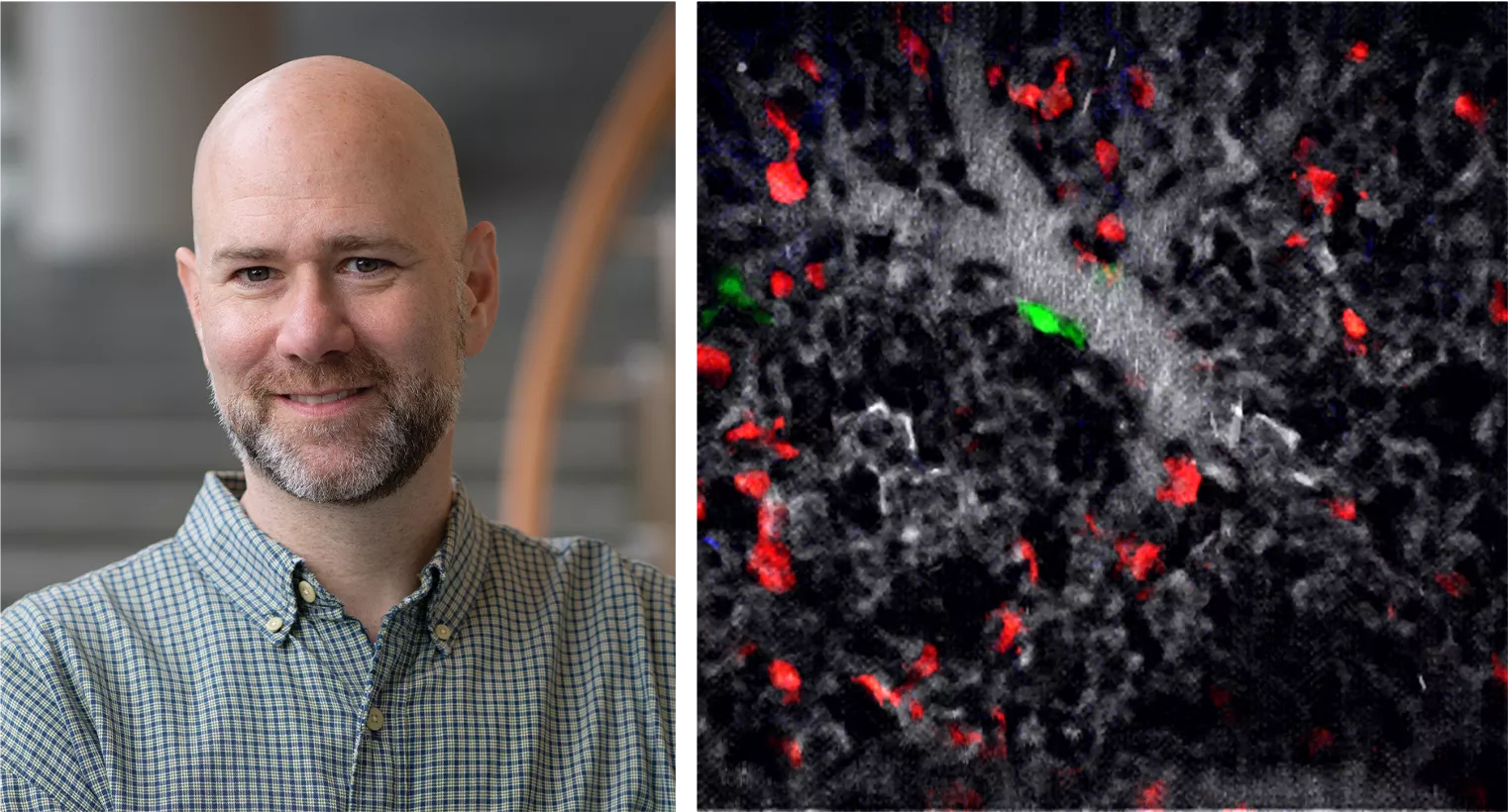
David Entenberg, PhD: Innovating Imaging Technology
Dr. David Entenberg, an associate professor of pathology, is at the forefront of developing advanced imaging technologies to better understand breast cancer metastasis. With a background in experimental quantum physics, he brings expertise in optical, mechanical, electrical, and software engineering to create tools that reveal the behavior of tumor cells in real-time. Dr. Entenberg has pioneered intravital imaging systems like the Window for High Resolution Intravital Imaging of the Lung (WHRIL), which visualizes interactions between cancer cells and the microenvironment within living tissues.
In a recent collaboration with Dr. Julio Aguirre-Ghiso, director of MECCC Cancer Dormancy Institute, and other MECCC members, Dr. Entenberg co-authored a study on lung macrophages, identifying their role in maintaining tumor cells in a dormant state. This research utilized the WHRIL technology to observe how different macrophages interact with disseminated tumor cells (DTCs), offering critical insights into preventing metastasis. The study was published in October 2024 in Cell.
Dr. Entenberg also serves as the co-director of Einstein's Gruss Lipper Biophotonics Center and as Director of Technological Development within the IIPCR. Additionally, he leads the IIPCR Computational Analysis of Biomarkers Program, which integrates his imaging technologies with computational methods to analyze biomarkers and improve cancer diagnosis and treatment. His innovative work is instrumental in translating basic science discoveries into clinical applications, aiming to disrupt metastasis pathways early on.
This collective research effort at the Montefiore Einstein Comprehensive Cancer Center strives to develop targeted strategies to stop metastasis at all stages.
Looking Ahead
Through the combined efforts of the clinical and basic science teams, Montefiore Einstein's Pathology Department is at the forefront of breast cancer care and research. By diagnosing complex cases, developing personalized treatment plans, and studying the mechanisms of cancer progression, these experts are transforming the landscape of breast cancer diagnosis and therapy.