News Releases
News Release
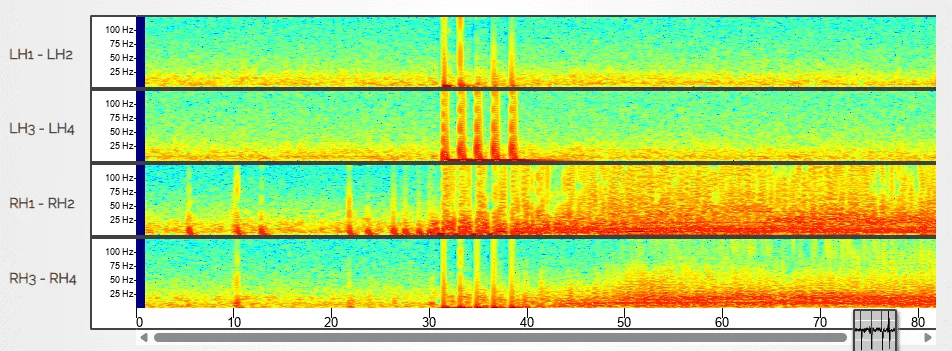
Pioneering Modeling & Therapeutic Discovery to Advance Care for Developmental Epileptic Encephalopathies
February 13, 2026
In the News
Header
Experts for Media
Header
Experts for Media
Research
Education & Training